PIPELINE
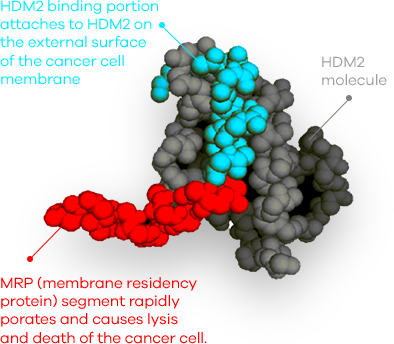
We are advancing OM-301 for the treatment of other blood cancers and solid tumors.
At the same time, we are exploring other similar peptides as well as novel immune-oncology approaches to deliver more effective therapeutics leveraging our unique mechanism of action for cancer treatment.
In early 2022, Oncolyze announced that the U.S. Food and Drug Administration (FDA) granted the company orphan drug designation for OM-301 for the treatment of acute myeloid leukemia (AML).